Sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl (>SO) functional group attached to two carbon atoms.
The double-bond character of the S−O bond may be accounted for by donation of electron density into C−S antibonding orbitals ("no-bond" resonance forms in valence-bond language).
[5] The S–O interaction has an electrostatic aspect, resulting in significant dipolar character, with negative charge centered on oxygen.
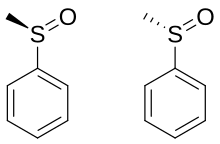
When the two organic residues are dissimilar, the sulfur atom is a chiral center, for example, in methyl phenyl sulfoxide.
The energy barrier required to invert this stereocenter is sufficiently high that sulfoxides are optically stable near room temperature.
[9][10] Symmetrical sulfoxides can be formed from a diorganylzinc compound and liquid sulfur dioxide.
[11] In addition to the oxidation routes, diaryl sulfoxides can be prepared by two Friedel–Crafts arylations of sulfur dioxide using an acid catalyst: Both aryl sulfinyl chlorides and diaryl sulfoxides can be also prepared from arenes through reaction with thionyl chloride in the presence of Lewis acid catalysts such as BiCl3, Bi(OTf)3, LiClO4, or NaClO4.
[14] The deoxygenation of dimethylsulfoxide is catalyzed by DMSO reductase, a molybdoenzyme:[15] The α-CH groups of alkyl sulfoxides are susceptible to deprotonation by strong bases, such as sodium hydride:[16] In the Pummerer rearrangement, alkyl sulfoxides react with acetic anhydride to give migration of the oxygen from sulfur to the adjacent carbon as an acetate ester.
Depending on the hard-soft properties of the metal, the sulfoxide binds through either the sulfur or the oxygen atom.