Sulfur isotope biogeochemistry
Measuring the abundance of sulfur stable isotopes in natural materials, like bacterial cultures, minerals, or seawater, can reveal information about these processes both in the modern environment and over Earth history.
The calculated value is called a "fractionation factor," and allows the effect of different processes on isotope distributions to be mathematically compared.
[11] The original CDT scale was based on a sample of the mineral troilite recovered from the Canyon Diablo meteorite at Meteor Crater, Arizona, US.
Samples are now measured in comparison to International Atomic Energy Agency (IAEA) reference materials, which are well-characterized, lab-prepared compounds with known δ34S values.
Sulfur is also an important component of biological material, including in the essential amino acids cysteine and methionine, the B vitamins thiamine and biotin, and the ubiquitous substrate coenzyme A.
[1] The effect of these metabolisms on sulfur isotopic composition of the reactants and products is also highly variable, depending on the rate of relevant reactions, availability of nutrients, diagenesis, and other biological, physical and environmental parameters.
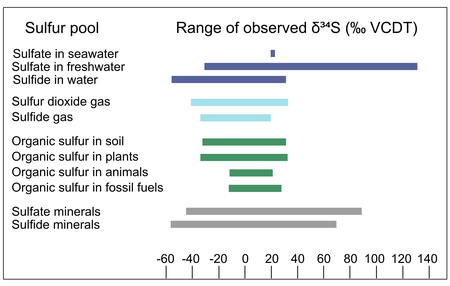
[25][28][29] As an example, the microbial reduction of sulfate to sulfide generally results in a 34S-depleted product, but the strength of this fractionation has been shown to range from 0 to 65.6‰ VCDT.
[31] Some sulfide minerals, including pyrite and galena, can form through thermochemical sulfate reduction, a process in which seawater sulfate trapped in seafloor rock is reduced to sulfide by geological heat as the rock is buried; this process generally fractionates sulfur more strongly than gypsum formation.
These organisms use sulfate reduction as an energy source as opposed to a way to synthesize new cell components, and remove the resulting sulfide as a waste product.
[64] Studies examining dozens of species of dissimilatory sulfate reducing microbes have observed sulfur isotope fractionations ranging from −65.6‰ to 0‰.
[71] Previous efforts to understand how sulfur metabolism and biosynthetic pathways relied on expensive labeling experiments using radioactive 35S.
In biological materials, sulfur is scarce relative to other organic elements like carbon and oxygen, introducing some additional difficulty in measuring its stable isotope composition.
[73] In order to reach detectable levels of 30 ng to 3 μg of elemental S to calculate reliable δ34S values, leaf tissue samples need to be between 2–5 mg.
The coupling of high-performance liquid chromatography (HPLC) with ICP-MS has been proposed as a way to test individual S-containing compounds.
[76] This organic sulfur is allocated into a diversity of compounds such as amino acids – namely cysteine (Cys) and methionine (Met) – proteins, cofactors, antioxidants, sulfate groups, Fe-S centers and secondary metabolites.
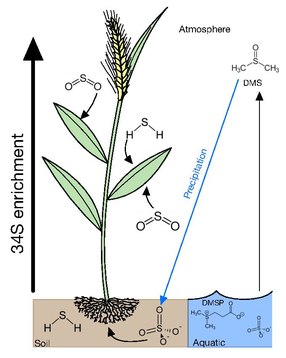
Most vegetation can acquire sulfur from gaseous atmospheric compounds or various ions either in soil solutions or water bodies.
The primary form of sulfur in soil is sulfate, which is transported upwards through the root system with minimal δ34S fractionation by 1–2‰.
Freshwater environments are more varied and subject to a multitude of sulfur inputs and outputs, including atmospheric deposition, runoff, diagenesis of bedrock and the presence of microbial sulfate reducers (MSR).
[78] In these environments algae will preferentially acquire sulfur from HS− if present, rather than the more abundant sulfate, as sulfide can be readily incorporated into the direct formation of cysteine.
At the surface of the sea, this excess in sulfur is subsequently converted into dimethylsulfoniopropionate (DMSP) by algae as an osmolyte and a repellent against grazing.
Sulfide reoxidation and disproportionation are also thought to be major processes affecting the sulfur isotopic compositions of marine minerals and sediment porewater.
[81] Cysteine acts as the direct or indirect precursor to any other organic sulfur compounds in plants such as coenzyme-A, methionine, biotin, lipoic acid and glutathione.
[78] The carbon skeleton necessary for sulfur assimilation are provided by glycolysis (acetyl-CoA), respiration (aspartic acid, Asp, which derives from oxaloacetate) and photorespiration (serine, Ser).
Though there is minimal fractionation from the source sulfur of the total plant organic matter, in wheat, roots and stems are depleted from soil by 2‰ and leaves and grain are 2‰ enriched.
As sulfate moves through the plant system and is incorporated into biomass, the pool becomes enriched, giving organs such as leaves and grains higher δ34S values than earlier tissues.
[83] Signatures of mass-anomalous sulfur isotope fractionation preserved in the rock record have been an important piece of evidence for understanding the Great Oxidation Event, the sudden rise of oxygen on the ancient Earth.
[1] By measuring the sulfur isotopic composition of these preserved materials, scientists can reconstruct ancient biological processes and the environments where they occurred.
[90][29] Microbial dissimilatory sulfate reduction (MSR), an energy-yielding metabolism performed by bacteria in anoxic environments, is associated with an especially large fractionation factor.
[1][60] Many studies have investigated the δ34S values of ancient pyrite in order to understand past biological and environmental conditions.
[94] Some studies compare sulfur isotopes in pyrite to a second sulfur-containing material, like dissolved sulfate or preserved organic matter.