Methionine
As the precursor of other non-essential amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans.
Methionine is also involved in angiogenesis and various processes related to DNA transcription, epigenetic expression, and gene regulation.
As a consequence, methionine is often incorporated into the N-terminal position of proteins in eukaryotes and archaea during translation, although it can be removed by post-translational modification.
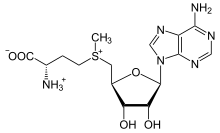
SAM-e is composed of an adenosyl molecule (via 5′ carbon) attached to the sulfur of methionine, therefore making it a sulfonium cation (i.e., three substituents and positive charge).
The sulfur acts as a soft Lewis acid (i.e., donor/electrophile) which allows the S-methyl group to be transferred to an oxygen, nitrogen, or aromatic system, often with the aid of other cofactors such as cobalamin (vitamin B12 in humans).
In bacteria, this is either regenerated by methylation or is salvaged by removing the adenine and the homocysteine, leaving the compound dihydroxypentandione to spontaneously convert into autoinducer-2, which is excreted as a waste product or quorum signal.
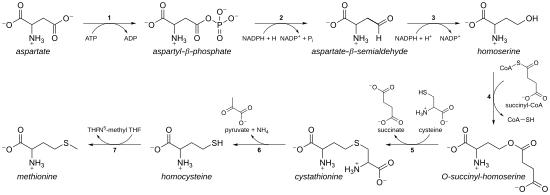
In plants and microorganisms, methionine biosynthesis belongs to the aspartate family, along with threonine and lysine (via diaminopimelate, but not via α-aminoadipate).
The main backbone is derived from aspartic acid, while the sulfur may come from cysteine, methanethiol, or hydrogen sulfide.
Cysteine is similarly produced, namely it can be made from an activated serine and either from homocysteine ("reverse transsulfurylation route") or from hydrogen sulfide ("direct sulfurylation route"); the activated serine is generally O-acetylserine (via CysK or CysM in E. coli), but in Aeropyrum pernix and some other archaea O-phosphoserine is used.
[citation needed] SAM-e serves as a methyl donor in many (2) methyltransferase reactions, and is converted to S-adenosylhomocysteine (SAH).
[citation needed] Homocysteine can also be remethylated using glycine betaine (N,N,N-trimethylglycine, TMG) to methionine via the enzyme betaine-homocysteine methyltransferase (E.C.2.1.1.5, BHMT).
The degradation of methionine is impaired in the following metabolic diseases:[citation needed] The industrial synthesis combines acrolein, methanethiol, and cyanide, which affords the hydantoin.
[20][21] The Food and Nutrition Board of the U.S. Institute of Medicine set Recommended Dietary Allowances (RDAs) for essential amino acids in 2002.
[25] Methionine raises the intracellular concentration of glutathione, thereby promoting antioxidant-mediated cell defense and redox regulation.