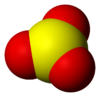
Sulfur trioxide
Sulfur trioxide exists in several forms: gaseous monomer, crystalline trimer, and solid polymer.
Sulfur trioxide is a solid at just below room temperature with a relatively narrow liquid range.
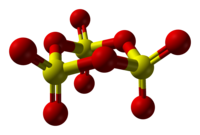
[9] Absolutely pure SO3 freezes at 16.8 °C to give the γ-SO3 form, which adopts the cyclic trimer configuration [S(=O)2(μ-O)]3.
[1] β-SO3, like the alpha form, is fibrous but of different molecular weight, consisting of an hydroxyl-capped polymer, but melts at 32.5 °C.
Sulfur trioxide is a potent sulfonating agent, i.e. it adds SO3 groups to substrates.
[14] For activated substrates, Lewis base adducts of sulfur trioxide are effective sulfonating agents.
A typical catalyst consists of vanadium pentoxide (V2O5) activated with potassium oxide K2O on kieselguhr or silica support.
Sulfur trioxide can be prepared in the laboratory by the two-stage pyrolysis of sodium bisulfate.
Sodium pyrosulfate is an intermediate product:[17] The latter occurs at much lower temperatures (45–60 °C) in the presence of catalytic H2SO4.
[17] Another two step method involving a salt pyrolysis starts with concentrated sulfuric acid and anhydrous tin tetrachloride: The advantage of this method over the sodium bisulfate one is that it requires much lower temperatures and can be done using normal borosilicate laboratory glassware without the risk of shattering.
B2O3 stabilized sulfur trioxide was traded by Baker & Adamson under the tradename "Sulfan" in the 20th century.