Supercapacitor
In 1966 researchers at Standard Oil of Ohio (SOHIO) developed another version of the component as "electrical energy storage apparatus", while working on experimental fuel cell designs.
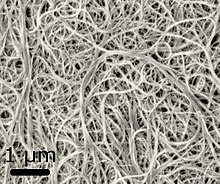
[11] Early electrochemical capacitors used two aluminum foils covered with activated carbon (the electrodes) that were soaked in an electrolyte and separated by a thin porous insulator.
In 1999 he defined the term "supercapacitor" to make reference to the increase in observed capacitance by surface redox reactions with faradaic charge transfer between electrodes and ions.
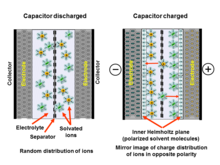
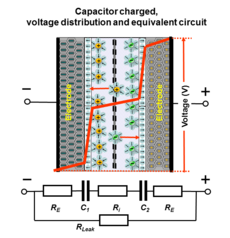
The double-layer charge forms a static electric field in the molecular layer of the solvent molecules in the IHP that corresponds to the strength of the applied voltage.
Applying a voltage at the electrochemical capacitor terminals moves electrolyte ions to the opposite polarized electrode and forms a double-layer in which a single layer of solvent molecules acts as separator.
Although conventional battery-type electrode materials also use chemical reactions to store charge, they show very different electrical profiles, as the rate of discharge is limited by the speed of diffusion.
Batteries offer lower purchase cost and stable voltage under discharge, but require complex electronic control and switching equipment, with consequent energy loss and spark hazard given a short.
[47][48] Carbide-derived carbons can exhibit high surface area and tunable pore diameters (from micropores to mesopores) to maximize ion confinement, increasing pseudocapacitance by faradaic H2 adsorption treatment.
However, a recent researcher, Li et al., from the University of Delaware found a facile and scalable approach to precipitate MnO2 on a SWNT film to make an organic-electrolyte based supercapacitor.
Electrolytes with organic solvents such as acetonitrile, propylene carbonate, tetrahydrofuran, diethyl carbonate, γ-butyrolactone and solutions with quaternary ammonium salts or alkyl ammonium salts such as tetraethylammonium tetrafluoroborate (N(Et)4BF4[82]) or triethyl (metyl) tetrafluoroborate (NMe(Et)3BF4) are more expensive than aqueous electrolytes, but they have a higher dissociation voltage of typically 1.35 V per electrode (2.7 V capacitor voltage), and a higher temperature range.
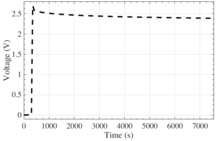
The capacitance value of a supercapacitor depends strongly on the measurement frequency, which is related to the porous electrode structure and the limited electrolyte's ion mobility.
These result in delayed current flow, reducing the total electrode surface area that can be covered with ions if polarity changes – capacitance decreases with increasing AC frequency.
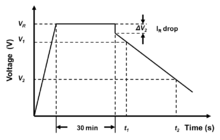
According to IEC/EN 62391-2, capacitance reductions of over 30%, or internal resistance exceeding four times its data sheet specifications, are considered "wear-out failures," implying that the component has reached end-of-life.
The following table shows differences among capacitors of various manufacturers in capacitance range, cell voltage, internal resistance (ESR, DC or AC value) and volumetric and gravimetric specific energy.
The first group offers greater ESR values of about 20 milliohms and relatively small capacitance of 0.1 to 470 F. These are "double-layer capacitors" for memory back-up or similar applications.
In applications with fluctuating loads, such as laptop computers, PDAs, GPS, portable media players, hand-held devices,[97] and photovoltaic systems, supercapacitors can stabilize the power supply.
[101] Power oscillations not only reduce the efficiency of the grid, but can cause voltage drops in the common coupling bus, and considerable frequency fluctuations throughout the entire system.
They are the sole power source for low energy applications such as automated meter reading (AMR)[105] equipment or for event notification in industrial electronics.
Supercapacitors can be used for micro grid storage to instantaneously inject power when the demand is high and the production dips momentarily, and to store energy in the reverse conditions.
They provide an immediate voltage buffer to compensate for quick changing power loads due to their high charge and discharge rate through an active control system.
The presence of the supercapacitor electrode alters the chemistry of the battery and affords it significant protection from sulfation in high rate partial state of charge use, which is the typical failure mode of valve regulated lead-acid cells used this way.
In 2005, aerospace systems and controls company Diehl Luftfahrt Elektronik GmbH chose supercapacitors to power emergency actuators for doors and evacuation slides used in airliners, including the Airbus 380.
The capacitors capture the braking energy of a full stop and deliver the peak current for starting the diesel engine and acceleration of the train and ensures the stabilization of line voltage.
[127] In 2012 tram operator Geneva Public Transport began tests of an LRV equipped with a prototype roof-mounted supercapacitor unit to recover braking energy.
[130] In August 2012 the CSR Zhuzhou Electric Locomotive corporation of China presented a prototype two-car light metro train equipped with a roof-mounted supercapacitor unit.
The supercapacitor and flywheel components, whose rapid charge-discharge capabilities help in both braking and acceleration, made the Audi and Toyota hybrids the fastest cars in the race.
The ability of supercapacitors to charge much faster than batteries, their stable electrical properties, broader temperature range and longer lifetime are suitable, but weight, volume and especially cost mitigate those advantages.
[151] As of 2013[update] all automotive manufacturers of EV or HEVs have developed prototypes that uses supercapacitors instead of batteries to store braking energy in order to improve driveline efficiency.
[152] Russian Yo-cars Ё-mobile series was a concept and crossover hybrid vehicle working with a gasoline driven rotary vane type and an electric generator for driving the traction motors.
[132] PSA Peugeot Citroën fit supercapacitors to some of its cars as part of its stop-start fuel-saving system, as this permits faster start-ups when the traffic lights turn green.












1. terminals, 2. safety vent, 3. sealing disc, 4. aluminum can, 5. positive pole, 6. separator, 7. carbon electrode, 8. collector, 9. carbon electrode, 10. negative pole

1. positive electrode, 2. negative electrode, 3. separator