Antimicrobial resistance
[11] Global initiatives, such as calls for international AMR treaties, emphasize coordinated efforts to limit misuse, fund research, and provide access to necessary antimicrobials in developing nations.
[15] WHO report released April 2014 stated, "this serious threat is no longer a prediction for the future, it is happening right now in every region of the world and has the potential to affect anyone, of any age, in any country.
[30] Self-medication by consumers is defined as "the taking of medicines on one's own initiative or on another person's suggestion, who is not a certified medical professional", and it has been identified as one of the primary reasons for the evolution of antimicrobial resistance.
[56] Important contributing factors are through "antibiotic residues", "industrial effluents", " agricultural runoffs", "heavy metals", "biocides and pesticides" and "sewage and wastewater" that create reservoirs for resistant genes and bacteria that facilitates the transfer of human pathogens.
Instead, there have been suggestions that when modern pathogenic bacteria interact with the ancient ones, they may, through horizontal gene transfer, pick up genetic sequences which are associated with antimicrobial resistance, exacerbating an already difficult issue.
Further detail and attention is still needed in order to recognize and measure trends in resistance on the international level; the idea of a global tracking system has been suggested but implementation has yet to occur.
It investigates the effectiveness of shorter versus longer antibiotic regimens for respiratory tract infections (RTIs) in a UK secondary care setting, emphasizing the need for evidence-based prescribing practices to optimize patient outcomes and combat AMR.
Schools further amplify the spread of infections due to close contact and shared surfaces, underscoring the importance of hygiene practices like regular handwashing, covering coughs, and staying home when unwell.
The "Interagency Coordination Group on Antimicrobial Resistance" stated in 2018 that "the spread of pathogens through unsafe water results in a high burden of gastrointestinal disease, increasing even further the need for antibiotic treatment.
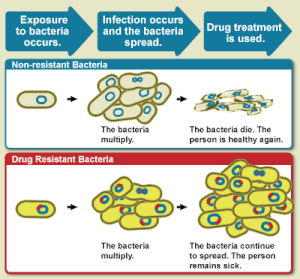
[116] Growing usage of antibiotics together with persistent infectious disease levels have led to a dangerous cycle in which reliance on antimicrobials increases while the efficacy of drugs diminishes.
[116] The proper use of infrastructure for water, sanitation and hygiene (WASH) can result in a 47–72 percent decrease of diarrhea cases treated with antibiotics depending on the type of intervention and its effectiveness.
[92][67] Recent studies have shown that the prophylactic use of "non-priority" or "non-clinically relevant" antimicrobials in feeds can potentially, under certain conditions, lead to co-selection of environmental AMR bacteria with resistance to medically important antibiotics.
In 2000, the FDA announced their intention to revoke approval of fluoroquinolone use in poultry production because of substantial evidence linking it to the emergence of fluoroquinolone-resistant Campylobacter infections in humans.
This global action plan developed by the World Health Organization was created to combat the issue of antimicrobial resistance and was guided by the advice of countries and key stakeholders.
The WHO's global action plan is composed of five key objectives that can be targeted through different means, and represents countries coming together to solve a major problem that can have future health consequences.
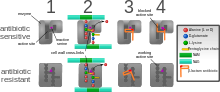
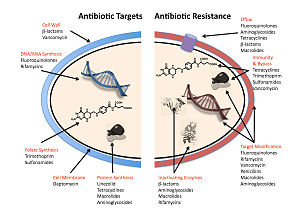
In addition, a number of resistance mechanisms depend on up-regulation of selected genes (for instance reflux pumps) rather than defined mutations that are amenable to molecular detection.
"[196][197] Without the creation of new and stronger antibiotics an era where common infections and minor injuries can kill, and where complex procedures such as surgery and chemotherapy become too risky, is a very real possibility.
[205] For the United States 2016 budget, U.S. president Barack Obama proposed to nearly double the amount of federal funding to "combat and prevent" antibiotic resistance to more than $1.2 billion.
According to the Centers for Disease Control and Prevention's 2023 Report on Antibiotic Resistance Threats, over 2.8 million antibiotic-resistant infections occur in the U.S. each year, leading to at least 35,000 deaths annually.
[208] Among the most concerning resistant pathogens are Carbapenem-resistant Enterobacteriaceae (CRE), Methicillin-resistant Staphylococcus aureus (MRSA), and Clostridioides difficile (C. diff), all of which continue to be responsible for severe healthcare-associated infections (HAIs).
In order to guide appropriate use of antibiotics and prevent the evolution and spread of antimicrobial resistance, diagnostic tests that provide clinicians with timely, actionable results are needed.
In 2017, scientists from Uppsala University in Sweden published a method[220] that applies principles of microfluidics and cell tracking, to monitor bacterial response to antibiotics in less than 30 minutes overall manipulation time.
Serum procalcitonin measurement has been shown to reduce mortality rate, antimicrobial consumption and antimicrobial-related side-effects in patients with respiratory infections, but impact on AMR has not yet been demonstrated.
In a phase II trial, a bivalent vaccine of capsular proteins 5 & 8 was tested in 1804 hemodialysis patients with a primary fistula or synthetic graft vascular access.
[231] Numerous investigators have suggested that a multiple-antigen vaccine would be more effective, but a lack of biomarkers defining human protective immunity keep these proposals in the logical, but strictly hypothetical arena.
[235] This phenomenon can be used to select against resistant bacteria using an approach termed collateral sensitivity cycling, which has recently been found to be relevant in developing treatment strategies for chronic infections caused by Pseudomonas aeruginosa.
[247] In 2011, Pfizer, one of the last major pharmaceutical companies developing new antibiotics, shut down its primary research effort, citing poor shareholder returns relative to drugs for chronic illnesses.
[254] The discovery and development of new antimicrobial agents has been facilitated by regulatory advances, which have been principally led by the European Medicines Agency (EMA) and the Food and Drug Administration (FDA).
[36] The bone regeneration material bioactive glass S53P4 has shown to effectively inhibit the bacterial growth of up to 50 clinically relevant bacteria including MRSA and MRSE.
[263] One of the key tools identified by the WHO and others for the fight against rising antimicrobial resistance is improved surveillance of the spread and movement of AMR genes through different communities and regions.