Superhard material
As a result of their unique properties, these materials are of great interest in many industrial areas including, but not limited to, abrasives, polishing and cutting tools, disc brakes, and wear-resistant and protective coatings.
Diamond demonstrates both high thermal conductivity and electrically insulating properties, and much attention has been put into finding practical applications of this material.
In this way, metals with high bulk moduli but low hardness are coordinated with small covalent-forming atoms to produce superhard materials.
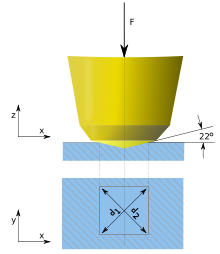
Measuring the mechanical hardness of materials changed to using a nanoindenter (usually made of diamond) and evaluating bulk moduli, and the Brinell, Rockwell, Knoop, and Vickers scales have been developed.
The incompressibility of a material is quantified by the bulk modulus B, which measures the resistance of a solid to volume compression under hydrostatic stress as B = −Vdp/dV.
Bulk moduli was the first major test of hardness and originally shown to be correlated with the molar volume (Vm) and cohesive energy (Ec) as B ~ Ec/Vm.
Bulk modulus was believed to be a direct measure of a material's hardness but this no longer remains the dominant school of thought.
For example, some alkali and noble metals (Pd, Ag) have anomalously high ratio of the bulk modulus to the Vickers or Brinell hardness.
If a material contains highly directional bonds, the shear modulus will increase and give a low Poisson ratio.
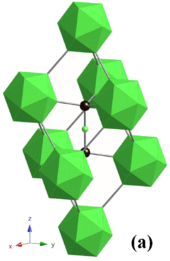
The inherent spatial separation of these subunits causes the formation of grains, which are visible by the unaided eye due to the light absorption and scattering properties of the material.
They achieve twins with an average thickness of 5 nm using a precursor of onion[clarification needed] carbon nanoparticles subjected to high temperature and pressure.
Higher thermal stability is relevant to industrial applications such as cutting tools, where high temperatures can lead to rapid diamond degradation.
Cubic boron nitride or c-BN was first synthesized in 1957 by Robert H. Wentorf at General Electric, shortly after the synthesis of diamond.
[11] The yield of c-BN is lower and substantially slower compared to diamond's synthetic route due to the complicated intermediate steps.
[33] Due to its great chemical and mechanical robustness, c-BN has widespread application as an abrasive, such as on cutting tools and scratch resistant surfaces.
[34] By modification, Borazon, a US brand name of c-BN, is used in industrial applications to shape tools, as it can withstand temperatures greater than 2,000 °C.
[36] Despite two decades of pursuit of this compound, no synthetic sample of C3N4 has validated the hardness predictions; this has been attributed to the difficulty in synthesis and the instability of C3N4.
It is also possible to make compounds containing B-C-O, B-O-N, or B-C-O-N under high pressure, but their synthesis would expect to require a complex chemistry and in addition, their elastic properties would be inferior to that of diamond.
They are expected to be thermally and chemically more stable than diamond, and harder than c-BN, and would therefore be excellent materials for high speed cutting and polishing of ferrous alloys.
It is unclear whether the synthesis products are diamond-like solid solutions between carbon and boron nitride or just mechanical mixtures of highly dispersed diamond and c-BN.
[9][40] Unlike carbon-based systems, metal borides can be easily synthesized in large quantities under ambient conditions, an important technological advantage.
An example synthesis of this material is the flux method, which is conducted by placing rhenium metal and amorphous boron in an alumina crucible with excess aluminium.
The crucible is placed in an alumina tube, inserted into a resistively heated furnace with flowing argon gas and sintered at 1,400 °C for several hours.
The density of states for ReB2 has one of the lowest values among the metal borides, indicating strong covalent bonding and high hardness.
[48][49][51] The discovery of superhard tungsten tetraboride is further evidence for the promising design approach of covalently bonding incompressible transition metals with boron.
However, researchers have been able to push WB2 into the superhard regime through minority additions of other transition metals such as niobium and tantalum in the crystal structure.
[54] This mechanism of hardness enhancement is called solid solution strengthening and arises because atoms of different sizes are incorporated into the parent lattice to impede dislocation motion.
Focus on synthesizing nano superhard materials is around minimizing microcracks occurring within the structure through grain boundary hardening.
Grain boundary strengthening is described by the Hall-Petch equation[57] Here σc is the critical fracture stress, d the crystallite size and σ0 and kgb are constants.
Besides grain boundary strengthening, much attention has been put into building microheterostructures, or nanostructures of two materials with very large differences in elastic moduli.