Synthesis of precious metals
The synthesis of precious metals involves the use of either nuclear reactors or particle accelerators to produce these elements.
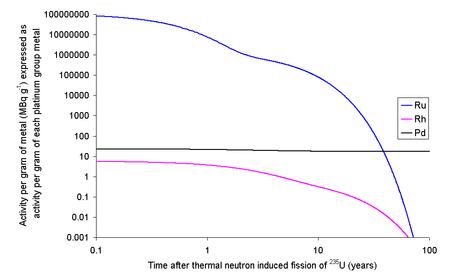
106Ru has a half-life of about 373 days, meaning that if the fuel is left to cool for 5 years before reprocessing only about 3% of the original quantity will remain; the rest will have decayed.
Such transmutation is possible in particle accelerators or nuclear reactors, although the production cost is estimated to be a trillion times the market price of gold.
[5] In 1924, a German scientist, Adolf Miethe, reported achieving the same feat, but after various replication attempts around the world, it was deemed an experimental error.
Seaborg's technique was far too expensive to enable the routine manufacture of gold but his work is the closest yet to emulating an aspect of the mythical Philosopher's stone.