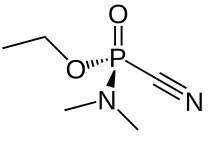
Tabun (nerve agent)
By irreversibly phosphorylating the enzyme,[2] Tabun accomplishes a constant and involuntary contraction of the affected muscles, as the acetylcholine is not recycled and continues to build up within the cleft.
[11] Tabun was made on an industrial scale by Germany during World War II, based on a process developed by Gerhard Schrader.
The symptoms of exposure include:[13][14][15] nervousness/restlessness, miosis (contraction of the pupil), rhinorrhea (runny nose), excessive salivation, dyspnea (difficulty in breathing due to bronchoconstriction/secretions), sweating, bradycardia (slow heartbeat), loss of consciousness, convulsions, flaccid paralysis, loss of bladder and bowel control, apnea (breathing stopped) and lung blisters.
[18] Research into ethyl dialkylaminocyanophosphonate began in the late 19th century, In 1898, Adolph Schall, a graduate student at the University of Rostock under professor August Michaelis, synthesised the diethylamino analog of tabun, as part of his PhD thesis Über die Einwirkung von Phosphoroxybromid auf secundäre aliphatische Amine.
[19] However, Schall incorrectly identified the structure of the substance as an imidoether, and Michaelis corrected him in a 1903 article in Liebigs Annalen, Über die organischen Verbindungen des Phosphors mit dem Stickstoff.
Tabun became the first nerve agent known after a property of this chemical was discovered by pure accident in late December 1936[16][13][20][21][22] by German researcher Gerhard Schrader.
[22] Schrader was experimenting with a class of compounds called organophosphates, which kill insects by interrupting their nervous systems, to create a more effective insecticide for IG Farben, a German chemical and pharmaceutical industry conglomerate, at Elberfeld.
[23] During World War II, as part of the Grün 3 program, a plant for the manufacture of tabun was established at Dyhernfurth (now Brzeg Dolny, Poland), in 1939.
[citation needed] The plant initially produced shells and aerial bombs using a 95:5 mix of tabun and chlorobenzene, designated "Variant A".
[citation needed] During the Nuremberg Trials, Albert Speer, Minister of Armaments and War Production for the Third Reich, testified that he had planned to kill Adolf Hitler in early 1945 by introducing tabun into the Führerbunker ventilation shaft.
[24] He said his efforts were frustrated by the impracticality of tabun and his lack of ready access to a replacement nerve agent,[24] and also by the unexpected construction of a tall chimney that put the air intake out of reach.
[citation needed] The German magazine Spiegel reported in 2007 that after World War II, "the United States dumped around half a million Tabun bombs in the Skagerrak in the northern Baltic" sea.
[26] Since GA is much easier to produce than the other G-series weapons[citation needed] and the process is comparatively widely understood, countries that develop a nerve agent capability but lack advanced industrial facilities often start by producing GA.[citation needed] During the Iran–Iraq War of 1980 to 1988, Iraq employed quantities of chemical weapons against Iranian ground forces.