Temperature
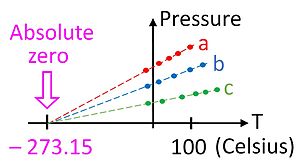
Experimentally, absolute zero can be approached only very closely; it can never be reached (the lowest temperature attained by experiment is 38 pK or 38 trillionths of a Kelvin).
Referring to the Boltzmann constant, to the Maxwell–Boltzmann distribution, and to the Boltzmann statistical mechanical definition of entropy, as distinct from the Gibbs definition,[5] for independently moving microscopic particles, disregarding interparticle potential energy, by international agreement, a temperature scale is defined and said to be absolute because it is independent of the characteristics of particular thermometric substances and thermometer mechanisms.
[citation needed] Historically, the temperature of the triple point of water was defined as exactly 273.16 K. Today it is an empirically measured quantity.
[8][9] Empirically based temperature scales rely directly on measurements of simple macroscopic physical properties of materials.
For example, the length of a column of mercury, confined in a glass-walled capillary tube, is dependent largely on temperature and is the basis of the very useful mercury-in-glass thermometer.
It is calibrated through the internationally agreed and prescribed value of the Boltzmann constant,[6][7] referring to motions of microscopic particles, such as atoms, molecules, and electrons, constituent in the body whose temperature is to be measured.
In an ideal gas, and in other theoretically understood bodies, the Kelvin temperature is defined to be proportional to the average kinetic energy of non-interactively moving microscopic particles, which can be measured by suitable techniques.
The Bose-Einstein law for this case indicates that the noise-power is directly proportional to the temperature of the resistor and to the value of its resistance and to the noise bandwidth.
Then the reference temperature, that of the triple point, was defined to be exactly 273.16 K. Since May 2019, that value has not been fixed by definition but is to be measured through microscopic phenomena, involving the Boltzmann constant, as described above.
Alternatively thinking, the ideal gas law, refers to the limit of infinitely high temperature and zero pressure; these conditions guarantee non-interactive motions of the constituent molecules.
[14] It is possible to measure the average kinetic energy of constituent microscopic particles if they are allowed to escape from the bulk of the system, through a small hole in the containing wall.
The other reason is that its zero is, in a sense, absolute, in that it indicates absence of microscopic classical motion of the constituent particles of matter, so that they have a limiting specific heat of zero for zero temperature, according to the third law of thermodynamics.
Nevertheless, a thermodynamic temperature does in fact have a definite numerical value that has been arbitrarily chosen by tradition and is dependent on the property of particular materials; it is simply less arbitrary than relative "degrees" scales such as Celsius and Fahrenheit.
For the Kelvin scale since May 2019, by international convention, the choice has been made to use knowledge of modes of operation of various thermometric devices, relying on microscopic kinetic theories about molecular motion.
The numerical scale is settled by a conventional definition of the value of the Boltzmann constant, which relates macroscopic temperature to average microscopic kinetic energy of particles such as molecules.
For the study by methods of classical irreversible thermodynamics, a body is usually spatially and temporally divided conceptually into 'cells' of small size.
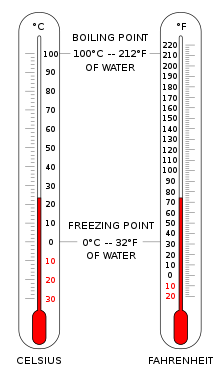
In most of the world (except for Belize, Myanmar, Liberia and the United States), the Celsius scale is used for most temperature measuring purposes.
[69] For instance, Fang and Ward were some of the first authors to successfully report temperature discontinuities of as much as 7.8 K at the surface of evaporating water droplets.
[69][76] Temperature discontiuities, rather than merely being anomalies, have actually substantially improved our understanding and predictive abilities pertaining to heat transfer at small scales.
[69][73][74][75][76] Historically, there are several scientific approaches to the explanation of temperature: the classical thermodynamic description based on macroscopic empirical variables that can be measured in a laboratory; the kinetic theory of gases which relates the macroscopic description to the probability distribution of the energy of motion of gas particles; and a microscopic explanation based on statistical physics and quantum mechanics.
In monatomic perfect gases and, approximately, in most gas and in simple metals, the temperature is a measure of the mean particle translational kinetic energy, 3/2 kBT.
The microscopic definition of temperature is only meaningful in the thermodynamic limit, meaning for large ensembles of states or particles, to fulfill the requirements of the statistical model.
The thermal energy may be partitioned into independent components attributed to the degrees of freedom of the particles or to the modes of oscillators in a thermodynamic system.
Using a sophisticated symmetry argument,[84] Boltzmann deduced what is now called the Maxwell–Boltzmann probability distribution function for the velocity of particles in an ideal gas.
[86] This direct proportionality between temperature and mean molecular kinetic energy is a special case of the equipartition theorem, and holds only in the classical limit of a perfect gas.
When two otherwise isolated bodies are connected together by a rigid physical path impermeable to matter, there is the spontaneous transfer of energy as heat from the hotter to the colder of them.
Sometimes the zeroth law is stated to include the existence of a unique universal hotness manifold, and of numerical scales on it, so as to provide a complete definition of empirical temperature.
[60] To be suitable for empirical thermometry, a material must have a monotonic relation between hotness and some easily measured state variable, such as pressure or volume, when all other relevant coordinates are fixed.
This relationship suggests the existence of a state function, S, whose change characteristically vanishes for a complete cycle if it is defined by where the subscript indicates a reversible process.
The finite quantum grand canonical ensemble,[94] obtained under the hypothesis of ergodicity and orthodicity,[95] allows expressing the generalized temperature from the ratio of the average time of occupation