SI base unit
The units and their physical quantities are the second for time, the metre (sometimes spelled meter) for length or distance, the kilogram for mass, the ampere for electric current, the kelvin for thermodynamic temperature, the mole for amount of substance, and the candela for luminous intensity.
The SI base units form a set of mutually independent dimensions as required by dimensional analysis commonly employed in science and technology.
Since the redefinition of the metre in 1960, the kilogram had been the only base unit still defined directly in terms of a physical artefact, rather than a property of nature.
This led to a number of the other SI base units being defined indirectly in terms of the mass of the same artefact; the mole, the ampere, and the candela were linked through their definitions to the mass of the International Prototype of the Kilogram, a roughly golfball-sized platinum–iridium cylinder stored in a vault near Paris.
The 21st General Conference on Weights and Measures (CGPM, 1999) placed these efforts on an official footing, and recommended "that national laboratories continue their efforts to refine experiments that link the unit of mass to fundamental or atomic constants with a view to a future redefinition of the kilogram".

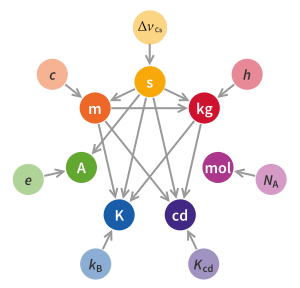
| Symbol | Name | Base quantity |
|---|---|---|
| s | second | time |
| m | metre | length |
| kg | kilogram | mass |
| A | ampere | electric current |
| K | kelvin | thermodynamic temperature |
| mol | mole | amount of substance |
| cd | candela | luminous intensity |