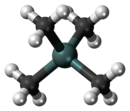
Tetramethyltin
[3] In tetramethyltin, the metal surrounded by four methyl groups in a tetrahedral structure is a heavy analogue of neopentane.
These methyltin chlorides are prepared via the so-called Kocheshkov redistribution reaction.
Di- and trimercaptotin compounds are used to inhibit the dehydrochlorination, which is the pathway for photolytic and thermal degradation of PVC.
[3] Tetramethyltin decomposes in the gas phase at about 277 °C; (CH3)4Sn vapor reacts with silica to give a (CH3)3Sn-grafted solid.
[4] In organic synthesis, tetramethyltin undergoes palladium-catalyzed coupling reactions with acid chlorides to give methyl ketones:[5]