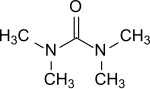
Tetramethylurea
[9] Tetramethylurea is suitable as a reaction medium for the polymerization of aromatic diacid chlorides (such as isophthalic acid) and aromatic diamines (such as 1,3-diaminobenzene (m-phenylenediamine)) to aramids such as poly (m-phenylene isophthalamide) (Nomex®)[10][11] The polymerization of 4-amino benzoic acid chloride hydrochloride in tetramethylurea provides isotropic viscous solutions of poly(p-benzamide) (PPB), which can be directly spun into fibers.
[12] In a tetramethylurea-LiCl mixture stable isotropic solutions can be obtained up to a PPB polymer concentration of 14%.
[13] Tetramethylurea also dissolves cellulose ester and swells other polymers such as polycarbonates, polyvinyl chloride or aliphatic polyamides, usually at elevated temperature.
If the reaction is carried out with acetobromoglucose and silver triflate/tetramethylurea at room temperature, then tetramethylurea reacts not only as a base, but also with the glycosyl to form a good isolable uroniumtriflates in 56% yield.
[19] The sensitization potential of tetramethylurea was shown to be low compared (non-sensitizing at 1% in LLNA testing according to OECD 429[20]).