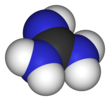
Guanidine
[4] A guanidine moiety also appears in larger organic molecules, including on the side chain of arginine.
[6] In 2013, the positions of the hydrogen atoms and their displacement parameters were accurately determined using single-crystal neutron diffraction.
Since the Middle Ages in Europe, guanidine has been used to treat diabetes as the active antihyperglycemic ingredient in French lilac.
Due to its long-term hepatotoxicity, further research for blood sugar control was suspended at first after the discovery of insulin.
Later development of nontoxic, safe biguanides led to the long-used first-line diabetes control medicine metformin, introduced to Europe in the 1950s & United States in 1995 and now prescribed to over 17 million patients per year in the US.
Guanidinium chloride is known to denature proteins with a linear relationship between concentration and free energy of unfolding.
In aqueous solutions containing 6 M guanidinium chloride, almost all proteins lose their entire secondary structure and become randomly coiled peptide chains.
[17] Gdx proteins, are highly selective for guanidinium and mono-substituted guanidinyl compounds and share an overlapping set of non-canonical substrates with drug exporter EmrE.