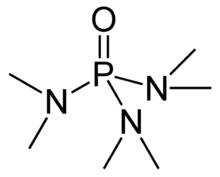
Hexamethylphosphoramide
Hexamethylphosphoramide, often abbreviated HMPA, is a phosphoramide (an amide of phosphoric acid) with the formula [(CH3)2N]3PO.
Compounds containing a nitrogen–phosphorus bond typically are degraded by hydrochloric acid to form a protonated amine and phosphate.
It improves the selectivity of lithiation reactions by breaking up the oligomers of lithium bases such as butyllithium.
Because HMPA selectively solvates cations, it accelerates otherwise slow SN2 reactions by generating more bare anions.
Both are strong hydrogen bond acceptors, and their oxygen atoms bind metal cations.