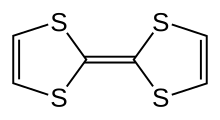
Tetrathiafulvalene
Studies on these heterocyclic compound contributed to the development of molecular electronics, although no practical applications of TTF emerged.
TTF is related to the hydrocarbon fulvalene (H4C4C=CC4H4) by replacement of four CH groups with sulfur atoms.
For TTF itself, the synthesis begins with the cyclic trithiocarbonate H2C2S2C=S (1,3-dithiole-2-thione), which is S-methylated and then reduced to give H2C2S2CH(SCH3) (1,3-dithiole-2-yl methyl thioether), which is treated as follows:[4] Protonolysis of a thioether: Followed by deprotonation of the dithiolium cation with triethylamine: Bulk TTF itself has unremarkable electrical properties.
[6] Subsequently, the charge-transfer salt [TTF]TCNQ was shown to be a narrow band gap semiconductor.
Well studied analogues include tetramethyltetrathiafulvalene (Me4TTF), tetramethylselenafulvalenes (TMTSFs), and bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF, CAS [66946-48-3]).