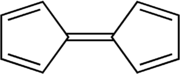
Fulvalene
Fulvalene is an unstable isomer of the more common benzenoid aromatic compounds naphthalene and azulene.
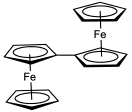
An earlier attempt at synthesis of fulvalene in 1951 by Pauson and Kealy resulted in the accidental discovery of ferrocene.
[1] Its synthesis was first reported in 1958 by E. A. Matzner, working under William von Eggers Doering.
Double deprotonation of the dihydrofulvalene with n-butyllithium gives the dilithio derivative, which is oxidized by oxygen.
Fulvalene was spectroscopically observed at −196 °C (77 K) from photolysis of diazocyclopentadiene, which induces dimerization of cyclopentadiene-derived carbenes.