Polyelectrolyte theory of the gene
[1] The polyelectrolyte theory of the gene was proposed by Steven A. Benner and Daniel Hutter in 2002[2] and has largely remained a theoretical framework astrobiologists have used to think about how life may be detected beyond Earth.
This idea was later linked by Benner [3][1] to Erwin Schrödinger's view of the gene as an "aperiodic crystal"[4] to make a robust, universally generalized concept of a genetic biopolymer—a biopolymer acting as a unit of inheritance in Darwinian evolution.
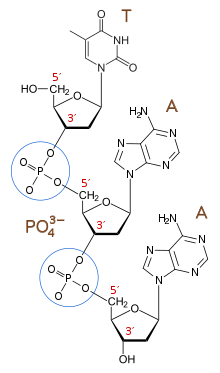
These phosphodiester linkages create the repeating negative charges on the molecule’s backbone that give DNA and RNA their polyelectrolyte nature.
In 2002, Steven A. Benner and Daniel Hutter identified the repeated charges in DNA's phosphodiester linkages as crucial to its function as a genetic biopolymer.
They proposed with the polyelectrolyte theory of the gene that repeated ionic charges—positive or negative—are a general requirement for all water-dissolved genetic biopolymers to undergo Darwinian evolution anywhere in the cosmos.
[2] This concept works in tandem with the view of the gene as an "aperiodic crystal" as proposed by Erwin Schrödinger in his 1944 book "What Is Life?".
[14] To work as a unit of inheritance, the genetic biopolymer must maintain shape and, therefore, physical and chemical consistency, regardless of the information the structure encodes.
This acronym gives scientists a shorthand way of describing the complex idea of a genetic biopolymer having the physical uniformity regardless of encoded information that allows it to be replicated.
Lab experiments conducted with non-electrolyte analogs of DNA and RNA initially inspired Benner and Hutton to publish on the polyelectrolyte theory of the gene.
[3] During the late ‘80s and '90s, scientists developed synthetic DNA-like molecules to bind to and silence unwanted mRNA gene products as a way to treat disease.
While initial experiments showed the sulfone analog to have very similar properties to DNA as a dimer—two nucleotides linked together—when longer sulfone analogs were synthesized, they folded, lost Watson–Crick base pair specificity, and had dramatic changes in physical properties due to small changes in nucleic acid sequence.
[2] In 2019, a group led by Philipp Holliger in Cambridge, England, developed non-electrolyte P-alkylphosphonate nucleic acids (phNA) DNA analogs that were able to undergo templated synthesis and directed evolution.
[22] This is around the length of the shortest naturally occurring gene, tRNA,[24] but is roughly an order of magnitude shorter than the genome of the smallest free-living organism.
This theory, combined with Schrödinger's view of a gene as an aperiodic crystal, provides a so-called "agnostic biosignature",[1] a sign of life that does not presuppose any biochemistry.
[1] Since the theorized genetic polyelectrolyte biomolecules could be charged either positively or negatively, as in the case of DNA and RNA, they can be concentrated in water with an electric field using electrophoresis or electrodialysis.
In addition, the molecules should be tested for the use of a limited number of building blocks arranged in a non-repeating fashion, an aperiodic crystal structure.
[28] Another suggested approach has been to use nanopore sequencing technology, although questions of whether the solar radiation experienced during transit and on-site would affect the functionality of the device remain.