Protein aggregation
[3][4] After synthesis, proteins typically fold into a particular three-dimensional conformation that is the most thermodynamically favorable: their native state.
Protein structures are stabilized by non-covalent interactions and disulfide bonds between two cysteine residues.
Extreme temperatures can weaken and destabilize the non-covalent interactions between the amino acid residues.
pHs outside of the protein's pH range can change the protonation state of the amino acids, which can increase or decrease the non-covalent interactions.
Oxidative stress can be caused by radicals such as reactive oxygen species (ROS).
These studies indicated that reducing the activity of insulin/IGF signaling (IIS), a prominent aging regulatory pathway protects from neurodegeneration-linked toxic protein aggregation.
The validity of this approach has been tested and confirmed in mammals as reducing the activity of the IGF-1 signaling pathway protected Alzheimer's model mice from the behavioral and biochemical impairments associated with the disease.
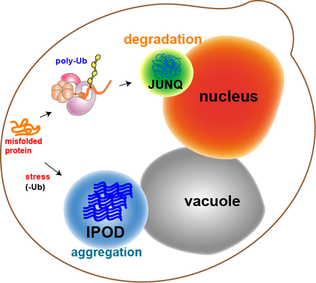
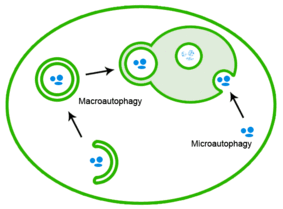
[15] Several studies have shown that cellular responses to protein aggregation are well-regulated and organized.
[16] From the macroscopic point of view, positron emission tomography tracers are used for certain misfolded proitein.
[17] Recently, a team of researchers led by Dr. Alessandro Crimi has proposed a machine learning method to predict future deposition in the brain.
This provides a natural selection mechanism for reducing protein aggregates in the bacterial population.
Different mutates of the same protein may form aggresomes of different morphologies, ranging from diffuse dispersion of soluble species to large puncta, which in turn bear different pathogenicity.
HDAC6 can then bind to the ubiquitin and the motor protein dynein to bring the marked aggregates to the microtubule organizing center (MTOC).
Hsp100 proteins have aromatic pore loops that are used for threading activity to disentangle single polypeptides.
[30] A quantitative assay has been used to determine that higher molecular weight species are responsible for the membrane permeation.
[31] It is known that protein aggregates in vitro can destabilize artificial phospholipid bilayers, leading to permeabilization of the membrane.
[citation needed] Protein aggregation is also a common phenomenon in the biopharmaceutical manufacturing process, which may pose risks to patients via generating adverse immune responses.