Thiocyanate
Thiocyanate used to be known as rhodanide (from a Greek word for rose) because of the red colour of its complexes with iron.
The other product depends on pH: in acid, it is hydrogen cyanide, presumably via HOSCN and with a sulfur dicyanide side-product; but in base and neutral solutions, it is cyanate.
Rhodanese catalyzes the reaction of sodium nitroprusside (like other cyanides) with thiosulfate to form the metabolite thiocyanate.
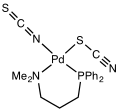
Experimental evidence leads to the general conclusion that class A metals (hard acids) tend to form N-bonded thiocyanate complexes, whereas class B metals (soft acids) tend to form S-bonded thiocyanate complexes.
Other factors, e.g. kinetics and solubility, are sometimes involved, and linkage isomerism can occur, for example [Co(NH3)5(NCS)]Cl2 and [Co(NH3)5(SCN)]Cl2.
[20] Both the iron and cobalt complexes can be extracted into organic solvents like diethyl ether or amyl alcohol.
[21] Phospholipids or some detergents aid the transfer of thiocyanatoiron into chlorinated solvents like chloroform and can be determined in this fashion.