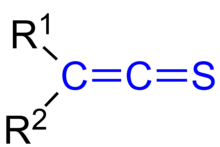Thioketene
In organic chemistry, thioketenes are organosulfur compounds analogous to ketenes with the general formula R2C=C=S, where R is alkyl or aryl.
[2] It has been suggested that thioketene could be involved in cell damage processes.
[3] Thioketenes can be stabilized by either steric protection or by electronic effects.
The violet color characteristic of thioketenes indicates the small HOMO-LUMO gap.
[5] These compound are prepared by treatment of the acid chloride with phosphorus pentasulfide as described by the following idealized equation: Bis(trifluoromethyl)thioketene ((CF3)2C=C=S) is an example of an electronically stabilized thioketene.