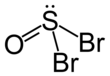
Thionyl bromide
[3] It is prepared by the action of hydrogen bromide on thionyl chloride,[4] although the corresponding reaction at higher pH (i.e. alkali bromides) proceeds only with difficulty:[1] Phosphorus trichlorodibromide (but not phosphorus pentabromide!)
Thionyl chlorobromide appears to be a key intermediate in these syntheses, but has not been isolated.
Unlike with thionyl chloride, stoichiometric bases are problematic activating agents, because free bromide anions decompose thionyl bromide to tribromide, sulfur dioxide, and sulfur.
[6][7] SOBr2 hydrolyzes readily in air to release dangerous fumes of sulfur dioxide and hydrogen bromide.
Decomposition to bromine and sulfur monoxide does not occur except at elevated temparatures.