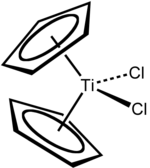
Titanocene dichloride
[2] It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.
The original synthesis by Wilkinson and Birmingham, using sodium cyclopentadienide,[4] is still commonly used:[5] It can also be prepared by using freshly distilled cyclopentadiene rather than its sodium derivative:[6] Focusing on the geometry of the Ti center, Cp2TiCl2 adopts a distorted tetrahedral geometry (counting Cp as a monodentate ligand).
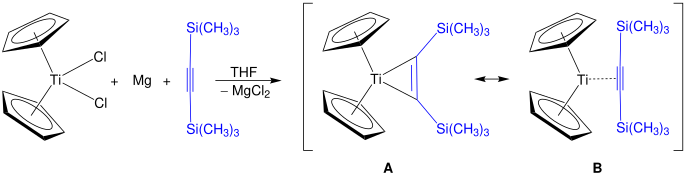
[14] Titanocene itself, TiCp2, is so highly reactive that it rearranges into a TiIII hydride dimer and has been the subject of much investigation.
[15][16] This dimer can be trapped by conducting the reduction of titanocene dichloride in the presence of ligands; in the presence of benzene, a fulvalene complex, μ(η5:η5-fulvalene)-di-(μ-hydrido)-bis(η5-cyclopentadienyltitanium), can be prepared and the resulting solvate structurally characterised by X-ray crystallography.
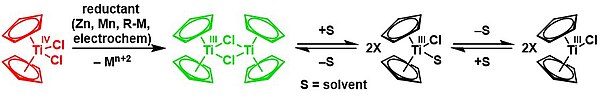
[15][16] Reduction with zinc gives the dimer of bis(cyclopentadienyl)titanium(III) chloride in a solvent-mediated chemical equilibrium:[21][22] Cp2TiCl2 is a precursor to TiII derivatives.
[26] Titanocene equivalents react with alkenyl alkynes followed by carbonylation and hydrolysis to form bicyclic cyclopentadienones, related to the Pauson–Khand reaction.
[27] A similar reaction is the reductive cyclization of enones to form the corresponding alcohol in a stereoselective manner.