Transalkylation
Motivation for using transalkylation reactions is based on a difference in production and demand for benzene, toluene, and xylenes.
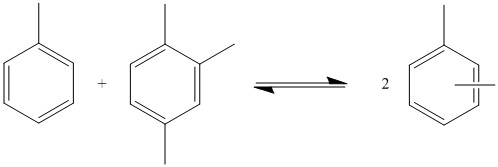
Since there is only a limited market for diethylbenzene, much of it is recycled by transalkylation to give ethylbenzene:[1] This type of reaction can also be performed with toluene and trimethylbenzene to produce xylene.
Side reactions in which alkanes are produced reduce the number of methyl groups available which decreases the M/R ratio.
[3] Transalkylation reactions of six to ten carbon methylated aromatics are often performed with the cofeed of hydrogen gas, over a zeolite based solid catalyst.
Aromatics molecules enter and exit these channels at different rates, also called diffusion.
In addition to their molecular sieving effect, zeolites have weakly bonded protons originated from its chemical composition.