Transuranium element
These two elements are generated by neutron capture in uranium ore with subsequent beta decays (e.g. 238U + n → 239U → 239Np → 239Pu).
The half-lives of these elements show a general trend of decreasing as atomic numbers increase.
[1] Transuranic elements are difficult and expensive to produce, and their prices increase rapidly with atomic number.
They are created through the bombardment of elements in a particle accelerator, in quantities on the atomic scale, and no method of mass creation has been found.
[7] Elements of the island of stability have potentially important military applications, including the development of compact nuclear weapons.
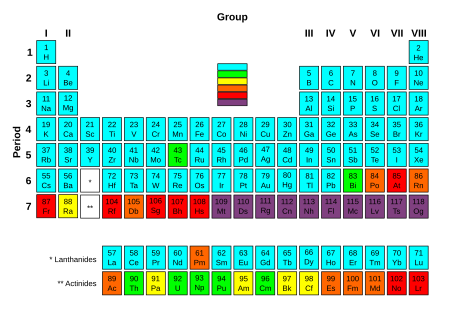
Elements which contain at least one stable isotope.
Slightly radioactive elements: the most stable isotope is very long-lived, with a half-life of over two million years.
Radioactive elements: the most stable isotope has half-life between 800 and 34,000 years.
Significantly radioactive elements: the most stable isotope has half-life between one day and 130 years.
Highly radioactive elements: the most stable isotope has half-life between several minutes and one day.
Extremely radioactive elements: the most stable known isotope has half-life less than several minutes.