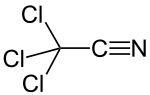
Trichloroacetonitrile
[2] Trichloroacetonitrile can be obtained by chlorination of acetonitrile on a zinc, copper and alkaline earth metal halide-impregnated activated carbon catalyst at 200–400 °C with a 54% yield.
[4] Like other halogenated acetonitriles, trichloroacetonitrile is produced from organic substances such as algae, humic acids and proteinaceous material in the disinfecting chlorination of water from natural sources.
[7] The substitution of all electronegative substituents in trichloroacetonitrile by nucleophilic attack of alkoxide anions produces orthocarbonic acid esters in high yield.
[9] Due to the mild reaction conditions, the Cl3CCN/PPh3 system is also suitable for the activation of carboxylic acids and their linkage with supported amino compounds to amides (peptides) in solid-phase syntheses.
For example, alcohols give O-alkyltrichloroacetimidates under basic catalysis in a direct and reversible addition,[15] which can be isolated as stable and less hydrolysis-sensitive adducts.
With primary and secondary amines, N-substituted trichloroacetamidines are formed in a smooth reaction with good yields, which can be purified by vacuum distillation and are obtained as colorless, malodorous liquids.
[16] Reaction with ammonia and then with anhydrous hydrogen chloride gives the solid trichloroacetamidine hydrochloride, the starting compound for the fungicide etridiazole.
[21] R. R. Schmidt and co-workers[22] have described the selective anomeric activation of O-protected hexopyranoses (glucose, galactose, mannose, glucosamine, galactosamine), hexofuranoses and pentopyranoses with trichloroacetonitrile in the presence of a base, as well as glycosylations under acid catalysis.