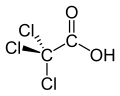
Trichloroacetic acid
[4] It is prepared by the reaction of chlorine with acetic acid in the presence of a suitable catalyst such as red phosphorus.
It is widely used in biochemistry for the precipitation of macromolecules, such as proteins, DNA, and RNA.
TCA and DCA are both used in cosmetic treatments (such as chemical peels and tattoo removal) and as topical medication for chemoablation of warts, including genital warts.
[7][8][9][10] According to the European Chemicals Agency, "This substance causes severe skin burns and eye damage, is very toxic to aquatic life and has long lasting toxic effects.
"[11] The discovery of trichloroacetic acid by Jean-Baptiste Dumas in 1839 delivered a striking example to the slowly evolving theory of organic radicals and valences.