Trichobilharzia regenti
[2] The species was originally described in 1998 in the Czech Republic[1] and afterwards it was detected also in other European countries, e.g. Denmark,[3] Belgium,[4] Germany,[5] France,[6] Iceland,[7] Poland,[8][9] Switzerland,[10] or Russia,[11] and even in Iran.
[12][13] For its unique neurotropic behaviour in vertebrate hosts, the host-parasite interactions are extensively studied in terms of molecular biology, biochemistry and immunology.
[25][26] A complete life cycle of T. regenti can be maintained under laboratory conditions using Radix lagotis and the Domestic duck (Anas platyrhynchos f. domestica) as intermediate and definitive hosts, respectively.
[29][30] Experiments with laboratory prepared recombinant form of the cysteine peptidase cathepsin B2 of T. regenti (TrCB2) confirmed its ability to cleave skin proteins (collagen, keratin and elastin).
[27] Based on recent observation by 3D imaging techniques (ultramicroscopy and micro-CT), schistosomula appear to migrate preferably through the white matter of the spinal cord in both birds and mammals.
[23][25] In case of repeated infections, the cellular infiltration is substantially elevated and the extensive inflammation may lead to formation of large abscesses or even epidermal and/or dermal necrosis.
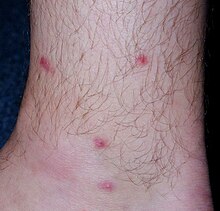
[23] In humans, the clinical symptoms of cercarial penetration consist of macules/papules formation at the sites where the parasite entered the skin accompanied by intensive itching.
This disease, caused not only by T. regenti but also by cercariae of other bird schistosome species, is called cercarial dermatitis (aka swimmer's itch).
[27] A cysteine peptidase cathepsin B1 of T. regenti (TrCB1) localised in intestines of migrating schistosomula is capable of myelin basic protein degradation, thus probably serving for nervous tissue digestion.
[27] This is underpinned by observations of leg paralysis only in immunocompromised hosts,[25][27] whereas in experiments with immunocompetent mouse strains, the infected animals did not reveal any neurological disorders.
[25][27][32] Recently, massive downregulation of neurophysiological pathways was suggested to be responsible for the motor dysfunction detected also in properly examined immunocompetent mice.
Lymphocytes from their skin draining lymph nodes produce IL-4 and IL-5 after stimulation with parasite antigens which shows Th2 polarization of host immune response.
[23] In a spinal cord, strong cellular immune response consisting of granulocytes, plasma cells, macrophages, and T-cells develops in immunocompetent mice especially around the damaged schistosomula.
CD3-deficient mice develop no or just mild inflammation which is accompanied by neurological symptoms due to mechanical damage caused to the nervous tissue.
[27] Murine astrocytes and microglia were shown to produce pro-inflammatory cytokines (IL-6 and TNF-α) and nitric oxide after in vitro exposure to parasite antigens, which supports their role in host immune response.
[36] High levels of IgG1 and IgG2b, but no IgG2a, specific to mostly protein epitopes of cercarial homogenate are detectable as long as 150 DPI in repeatedly infected mice.
[2][24] Majority of humans (82% of adults, 57% of children) who have experienced cercarial dermatitis (caused by undetermined species of bird schistosome) have increased levels of T. regenti antigen-specific IgG, but not IgE.