Toxocariasis
It is characterized by fever, eosinophilia, urticaria, enlarged lymph nodes, cough, bronchospasm, wheezing, abdominal pain, headaches, and/or hepatosplenomegaly.
Visceral larva migrans (VLM) is a more severe form of the disease; signs and symptoms depend on the specific organ system(s) involved.
Ocular larva migrans (OLM) is the third syndrome, manifesting as uveitis, endophthalmitis, visual impairment or even blindness in the affected eye.
[8] Signs and symptoms of covert toxocariasis are coughing, fever, abdominal pain, headaches, and changes in behavior and ability to sleep.
Children can present with pallor, fatigue, weight loss, anorexia, fever, headache, urticaria skin rash, cough, asthma, chest tightness, increased irritability, abdominal pain, nausea, and vomiting.
[7] Children are commonly diagnosed with pneumonia, bronchospasms, chronic pulmonary inflammation, hypereosinophilia, hepatomegaly, hypergammaglobulinaemia (IgM, IgG, and IgE classes), leukocytosis, and elevated anti-A and anti-B isohaemagglutinins.
[7][11] A light Toxocara burden is thought to induce a low immune response, allowing a larva to enter the host's eye.
[9] Young children who put contaminated objects in their mouths or eat dirt (pica) are at risk of developing symptoms.
[18] Eating undercooked rabbit, chicken, or sheep can lead to infection; encysted larvae in the meat can become reactivated and migrate through a human host, causing toxocariasis.
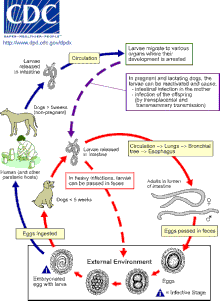
[7][16] T. canis females, specifically, are capable of producing up to 200,000 eggs a day that require 2–6 weeks minimum up to a couple months before full development into the infectious stage.
[9][16] Provided sufficient oxygen and moisture availability, Toxocara eggs can remain infectious for years, as their resistant outer shell enables protection from most environmental threats.
[20][21][22] However, as identified in a case study presented within the journal of helminthology, the second stage of larvae development poses strict vulnerabilities to certain environmental elements.
[20] Species include:[23] Dogs and foxes are the reservoir for Toxocara canis, but puppies and cubs pose the greatest risk of spreading the infection to humans.
[21][22] Adult T. canis are found only within dogs and foxes and the males are 4–6 cm in length, with a curved posterior end.
[22] Cats, dogs, and foxes can become infected with Toxocara through the ingestion of eggs or by transmission of the larvae from a mother to her offspring.
[16][25] Transmission to cats and dogs can also occur by ingestion of infected accidental hosts, such as earthworms, cockroaches, rodents, rabbits, chickens, or sheep.
The larvae mature into adults within the small intestine of a cat, dog, or fox, where mating and egg-laying occurs.
[9][22] In most adult dogs, cats and foxes, the full lifecycle does not occur, but instead second stage larvae encyst after a period of migration through the body.
[16][25] Second-stage larvae will also hatch in the small intestine of an accidental host, such as a human, after ingestion of infective eggs.
A group very actively involved in promoting a reduction of infections in dogs in the United States is the Companion Animal Parasite Council -- CAPC.
Since pregnant or lactating dogs and cats and their offspring have the highest, active parasitic load, these animals should be placed on a deworming program.
Hand washing before eating and after playing with pets, as well as after handling dirt will reduce the chances of ingesting Toxocara eggs.
[9] Finally, teaching children not to place nonfood items, especially dirt, in their mouths will drastically reduce the chances of infection.
[5] Toxocariasis has been named one of the neglected diseases of US poverty, because of its prevalence in Appalachia, the southern U.S., inner city settings, and minority populations.
[9][12][30][34] Visceral toxocariasis in humans can be treated with antiparasitic drugs such as albendazole or mebendazole, tiabendazole or diethylcarbamazine usually in combination with anti-inflammatory medications.
[12] Young children are at the greatest risk of infection because they play outside and tend to place contaminated objects and dirt in their mouths.
[9] The "excretory-secretory antigens of larvae ... released from their outer epicuticle coat [and] ... readily sloughed off when bound by specific antibodies" incite the host's immune response.
[9] The lighter infection in OLM is believed to stimulate a lower immune response and allow for the migration of a larva into the eye.
[38] Some treatments for infection with Toxocara cati include drugs designed to cause the adult worms to become partially anaesthetized and detach from the intestinal lining, allowing them to be excreted live in the feces.
These are frequently combined with the drug praziquantel which appears to cause the worm to lose its resistance to being digested by the host animal.