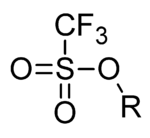
Triflate
In organic chemistry, triflate (systematic name: trifluoromethanesulfonate), is a functional group with the formula R−OSO2CF3 and structure R−O−S(=O)2−CF3.
Since alkyl triflates are extremely reactive in SN2 reactions, they must be stored in conditions free of nucleophiles (such as water).
A mild triflating reagent is phenyl triflimide or N,N-bis(trifluoromethanesulfonyl)aniline, where the by-product is [CF3SO2N−Ph]−.
An example is the Mukaiyama aldol addition reaction between benzaldehyde and the silyl enol ether of cyclohexanone with an 81% chemical yield.
[2] The corresponding reaction with the yttrium salt fails: Triflate is a commonly used weakly coordinating anion.