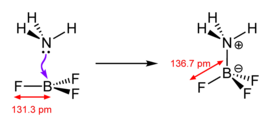Lewis acids and bases
Some sources indicate the Lewis base with a pair of dots (the explicit electrons being donated), which allows consistent representation of the transition from the base itself to the complex with the acid: A center dot may also be used to represent a Lewis adduct, such as Me3B·NH3.
Moreover, in some cases (e.g., sulfoxides and amine oxides as R2S → O and R3N → O), the use of the dative bond arrow is just a notational convenience for avoiding the drawing of formal charges.
Simplest are those that react directly with the Lewis base, such as boron trihalides and the pentahalides of phosphorus, arsenic, and antimony.
However, the methyl cation never occurs as a free species in the condensed phase, and methylation reactions by reagents like CH3I take place through the simultaneous formation of a bond from the nucleophile to the carbon and cleavage of the bond between carbon and iodine (SN2 reaction).
[8] Some of the most studied examples of such Lewis acids are the boron trihalides and organoboranes:[9] In this adduct, all four fluoride centres (or more accurately, ligands) are equivalent.
Monomeric BH3 does not exist appreciably, so the adducts of borane are generated by degradation of diborane: In this case, an intermediate B2H−7 can be isolated.
[12] Nearly all electron pair donors that form compounds by binding transition elements can be viewed ligands.
Thus, a large application of Lewis bases is to modify the activity and selectivity of metal catalysts.
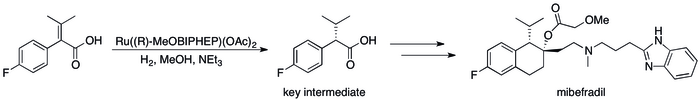
The industrial synthesis of the anti-hypertension drug mibefradil uses a chiral Lewis base (R-MeOBIPHEP), for example.
In this context hard implies small and nonpolarizable and soft indicates larger atoms that are more polarizable.
[citation needed] Many methods have been devised to evaluate and predict Lewis acidity.
The E and C parameters refer, respectively, to the electrostatic and covalent contributions to the strength of the bonds that the acid and base will form.
The equation is The W term represents a constant energy contribution for acid–base reaction such as the cleavage of a dimeric acid or base.
[15][16] and that single property scales are limited to a smaller range of acids or bases.
In 1923, Lewis wrote An acid substance is one which can employ an electron lone pair from another molecule in completing the stable group of one of its own atoms.
The strength of Lewis acid-base interactions, as measured by the standard enthalpy of formation of an adduct can be predicted by the Drago–Wayland two-parameter equation.
Lewis had suggested in 1916 that two atoms are held together in a chemical bond by sharing a pair of electrons.