Hexagonal crystal family
[1] In particular, there are crystals that have trigonal symmetry but belong to the hexagonal lattice (such as α-quartz).
The Bravais lattices in the hexagonal crystal family can also be described by rhombohedral axes.
[5] In practice, the hexagonal description is more commonly used because it is easier to deal with a coordinate system with two 90° angles.
The hexagonal crystal system consists of the 7 point groups that have a single six-fold rotation axis.
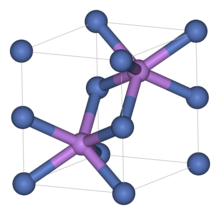
The wurtzite crystal structure is referred to by the Strukturbericht designation B4 and the Pearson symbol hP4.
The Hermann-Mauguin symbols in P63mc can be read as follows:[13] Among the compounds that can take the wurtzite structure are wurtzite itself (ZnS with up to 8% iron instead of zinc), silver iodide (AgI), zinc oxide (ZnO), cadmium sulfide (CdS), cadmium selenide (CdSe), silicon carbide (α-SiC), gallium nitride (GaN), aluminium nitride (AlN), boron nitride (w-BN) and other semiconductors.
Each of the two individual atom types forms a sublattice which is hexagonal close-packed (HCP-type).
The structure can also be described as an HCP lattice of zinc with sulfur atoms occupying half of the tetrahedral voids or vice versa.
[15] The structure can also be described as an HCP lattice of arsenic with nickel occupying each octahedral void.
Compounds adopting the NiAs structure are generally the chalcogenides, arsenides, antimonides and bismuthides of transition metals.