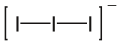Triiodide
The process is analogous to the reaction of S8 with sodium sulfide (which forms polysulfides) except that the higher polyiodides have branched structures.
In ionic compounds, the bond lengths and angles of triiodide vary depending on the nature of the cation.
Only in combination with large cations, e.g. a quaternary ammonium such as [N(CH3)4]+, may the triiodide remain roughly symmetrical.
[3] In solution phase, the bond lengths and angles of triiodide vary depending on the nature of solvent.
The triiodide ion is responsible for the well-known blue-black color which arises when iodine solutions interact with starch.
[11] It has been shown that the solid state photoreaction mechanism depends on the light wavelength, yielding fast recovery in a few picoseconds[12] or going through a two-stage process that involves the formation and break-up of a tetraiodide intermediate on longer timescales.
[14] Because of the presence of heavy iodine atoms and the well-calibrated chemical pathways, triiodide has also become a computational benchmark system for relativistic quantum chemistry.
[15] The redox reactions of triiodide and iodide has been proposed as critical steps in dye-sensitized solar cells.