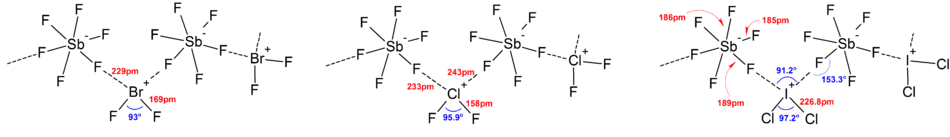Polyhalogen ions
Numerous polyhalogen ions have been found, with their salts isolated in the solid state and structurally characterized.
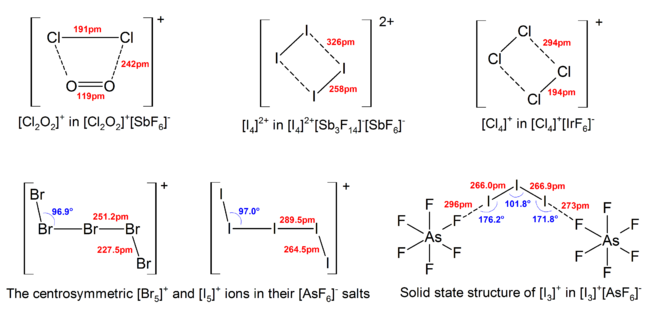
More deviations from the ideal VSEPR model were found in the solid state structures due to strong cation-anion interactions, which also complicates interpretation of vibrational spectroscopic data.
In the solid states, the polyiodides can interact with each other to form chains, rings, or even complicated two-dimensional and three-dimensional networks.
Linear or nearly-linear triatomic polyhalides have weaker and longer bonds compared with that in the corresponding diatomic interhalogen or halogen, consistent with the additional repulsion between atoms as the halide ion is added to the neutral molecule.
[7] The formation of polyhalogen ions can be viewed as the self-dissociation of their parent interhalogens or halogens: There are two general strategies for preparing polyhalogen cations: In some cases the Lewis acid (the fluoride acceptor) itself acts as an oxidant: Usually the first method is employed for preparing heteropolyhalogen cations, and the second one is applicable to both.
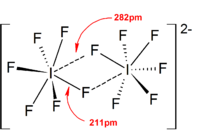
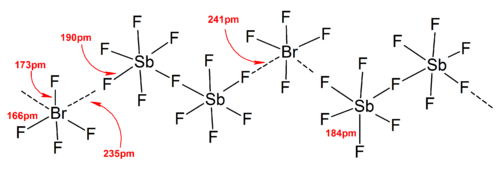
[8]: v1p294 In general, a large counter cation or anion (such as Cs+ and [SbF6]−) can help stabilize the polyhalogen ions formed in the solid state from lattice energy considerations, as the packing efficiency increases.
For example, [I2]+ is stable in fluoroantimonic acid (HF with 0.2 N SbF5, H0 = −20.65), but disproportionates to [I3]+, [I5]+ and I2 when weaker fluoride acceptors, like NbF5, TaF5 or NaF, are added instead of SbF5.
therefore the stability of the anions decrease in the order: Heteropolyhalogen ions with a coordination number larger than or equal to four can only exist with fluoride ligands.
The well-known starch-iodine complex has a deep blue color due to the linear [I5]− ions present in the amylose helix.
For example, [Cl2F]+ is unstable in solution and disproportionate completely in HF/SbF5 mixture even at 197 K: [I2]+ reversibly dimerizes at 193 K, and is observed as the blue color of paramagnetic [I2]+ dramatically shifts to the red-brown color of diamagnetic [I2]+, together with a drop in paramagnetic susceptibility and electrical conductivity when the solution is cooled to below 193 K:[2] The dimerization can be attributed to the overlapping of the half-filled π* orbitals in two [I2]+.
[2] Attempts to prepare ClF7 and BrF7 by fluorinating [ClF6]+ and [BrF6]+ using NOF have met with failure, because the following reactions occurred:[3] The anions are less reactive compared to the cations, and are generally weaker oxidants than their parent interhalogens.