Triple quadrupole mass spectrometer
[1] After coming into contact with Prof. Christie G. Enke and his then graduate student Richard Yost, Morrison's linear arrangement of the three quadrupoles probed the construction of the first triple-quadrupole mass spectrometer.
[1] In the years following, the first commercial triple-quadrupole mass spectrometer was developed at Michigan State University by Enke and Yost in the late 1970s.
[2] It was later found that the triple-quadrupole mass spectrometer could be utilized to study organic ions and molecules, thus expanding its capabilities as a tandem MS/MS technique.
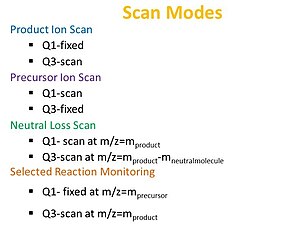
Both, the first mass analyzer and the collision cell are continuously exposed to ions from the source, in a time independent manner.
The collision cell, denoted as "q", is located between Q1 and Q3, is where fragmentation of the sample occurs in the presence of an inert gas like Ar, He, or N2.
Upon exiting the collision cell, the fragmented ions then travel onto the second quadrupole mass filter, Q3, where m/z selection can occur again.
[3] Employing the TQMS provides enhanced selectivity, better accuracy, and greater reproducibility; all of which are limited in single quadrupole mass analyzers.
[7] The triple quadrupole mass spectrometer allows for increased sensitivity and specificity yielding lower detection and quantitation limits.
[8] For these reasons, employment of the TQMS is a vital asset in the fields of drug metabolism, pharmacokinetics, environmental studies, and biological analyses.
By analyzing the rat’s urine or plasma with a triple quadrupole coupled to liquid chromatography, the concentration and fragmentation pattern of the new drug can be determined.