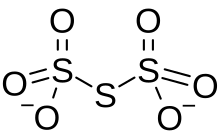
Trithionate
Trithionate is an oxyanion of sulfur with the chemical formula S3O2−6.
It is the conjugate base of trithionic acid.
[1] Dilute sodium hydroxide hydrolyzes S4N4 as follows, yielding sodium thiosulfate and sodium trithionate: Certain sulfate-reducing bacteria have been known to use the compound in respiration.
This article about chemical compounds is a stub.
You can help Wikipedia by expanding it.