Tsuji–Trost reaction
[4] The introduction of phosphine ligands led to improved reactivity and numerous asymmetric allylic alkylation strategies.
The ability to form carbon-carbon, carbon-nitrogen, and carbon-oxygen bonds under these conditions, makes this reaction very appealing to the fields of both medicinal chemistry and natural product synthesis.
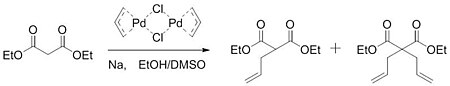
[5] Gaining insight from this work, Tsuji hypothesized that a similar activation could take place to form carbon-carbon bonds.
By reacting an allylpalladium chloride dimer with the sodium salt of diethyl malonate, the group was able to form a mixture of monoalkylated and dialkylated product.
While attempting to synthesize acyclic sesquiterpene homologs, Trost ran into problems with the initial procedure and was not able to alkylate his substrates.
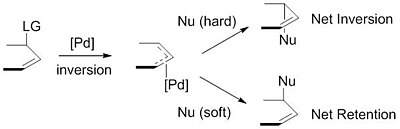
Starting with a zerovalent palladium species and a substrate containing a leaving group in the allylic position, the Tsuji–Trost reaction proceeds through the catalytic cycle outlined below.
The next step is oxidative addition in which the leaving group is expelled with inversion of configuration and a η3 π-allyl-PdII is created (also called ionization).
Importantly, these ligands can also instill chirality to the final product, making it possible for these reactions to be carried out asymmetrically as shown below.
[13][14][15] The reaction was originally developed with a palladium catalyst supported by the Trost ligand, although suitable conditions have greatly expanded since then.
Some of the most common nucleophiles include malonates, enolates, primary alkoxides, carboxylates, phenoxides, amines, azide, sulfonamides, imides, and sulfones.
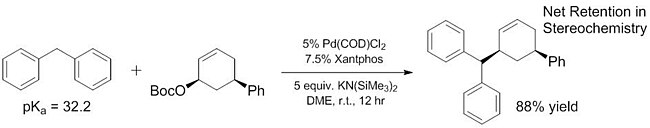
By increasing the scope of pronucleophiles that act as "soft" nucleophiles, these substrates can also be incorporated into enantioselective reactions using previously reported and well characterized methods.
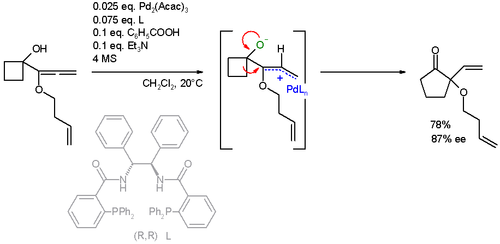
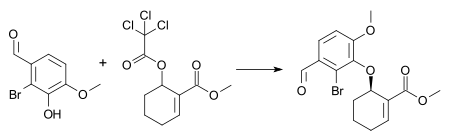
In this specific ring expansion the AAA reaction is also accompanied by a Wagner–Meerwein rearrangement:[24][25] The ability to form carbon-carbon, carbon-nitrogen, and carbon-oxygen bonds enantioselectively under mild conditions makes the Trost asymmetric allylic alkylation extremely appealing for the synthesis of complex molecules.
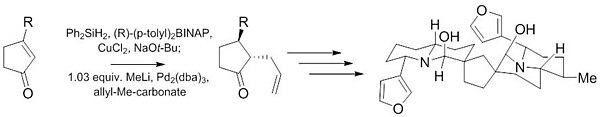
This recent work demonstrates the ability of this reaction to give highly diastereoselective (10:1) and enantioselective (97.5:2.5) products from achiral starting material with only a small amount of catalyst (1%).