Chirality
A chiral object and its mirror image are called enantiomorphs (Greek, "opposite forms") or, when referring to molecules, enantiomers.
The term was first used by Lord Kelvin in 1893 in the second Robert Boyle Lecture at the Oxford University Junior Scientific Club which was published in 1894: I call any geometrical figure, or group of points, 'chiral', and say that it has chirality if its image in a plane mirror, ideally realized, cannot be brought to coincide with itself.
The J, L, S and Z-shaped tetrominoes of the popular video game Tetris also exhibit chirality, but only in a two-dimensional space.
Many other familiar objects exhibit the same chiral symmetry of the human body, such as gloves, glasses (sometimes), and shoes.
In terms of point groups, all chiral figures lack an improper axis of rotation (Sn).
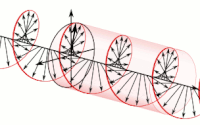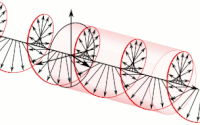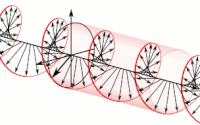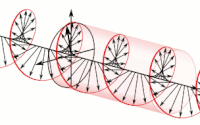
The handedness in both chirality and helicity relate to the rotation of a particle while it proceeds in linear motion with reference to the human hands.
Polarization of an electromagnetic wave is the property that describes the orientation, i.e., the time-varying direction and amplitude, of the electric field vector.
For example, the electric field vectors of left-handed or right-handed circularly polarized waves form helices of opposite handedness in space.
2D-chiral patterns, such as flat spirals, cannot be superposed with their mirror image by translation or rotation in two-dimensional space (a plane).
2D-chiral materials, which are also anisotropic and lossy exhibit different total transmission (reflection and absorption) levels for the same circularly polarized wave incident on their front and back.
Like the twist of a 2d-chiral pattern appears reversed for opposite directions of observation, 2d-chiral materials have interchanged properties for left-handed and right-handed circularly polarized waves that are incident on their front and back.
In particular left-handed and right-handed circularly polarized waves experience opposite directional transmission (reflection and absorption) asymmetries.
Instead, both effects can also occur when the propagation direction of the electromagnetic wave together with the structure of an (achiral) material form a chiral experimental arrangement.
[14] However, most absorbing chiral mirrors operate only in a narrow frequency band, as limited by the causality principle.
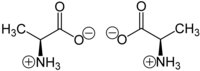
However, this d- and l- notation of distinguishing enantiomers does not say anything about the actual spatial arrangement of the ligands/substituents around the stereogenic center, which is defined as configuration.
[25] All of the known life-forms show specific chiral properties in chemical structures as well as macroscopic anatomy, development and behavior.
From chemical level (molecular scale), biological systems show extreme stereospecificity in synthesis, uptake, sensing, metabolic processing.
The chiral protein-making amino acids, which are translated through the ribosome from genetic coding, occur in the L form.
[28] Also, for artificial compounds, including medicines, in case of chiral drugs, the two enantiomers sometimes show remarkable difference in effect of their biological actions.
In case of penicillamine, the (S-isomer is used in the treatment of primary chronic arthritis, whereas the (R)-isomer has no therapeutic effect, as well as being highly toxic.
The naturally occurring plant form of alpha-tocopherol (vitamin E) is RRR-α-tocopherol whereas the synthetic form (all-racemic vitamin E, or dl-tocopherol) is equal parts of the stereoisomers RRR, RRS, RSS, SSS, RSR, SRS, SRR, and SSR with progressively decreasing biological equivalency, so that 1.36 mg of dl-tocopherol is considered equivalent to 1.0 mg of d-tocopherol.
A simple example is the coiling direction of any climber plant, which can grow to form either a left- or right-handed helix.
In anatomy, chirality is found in the imperfect mirror image symmetry of many kinds of animal bodies.
Remarkably, the related genus Dilatris also has chirally dimorphic flowers, but here both morphs occur on the same plant.