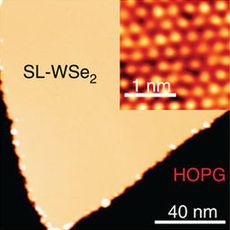
Tungsten diselenide
The two-dimensional lattice structure has W and Se arranged periodically in layers with hexagonal symmetry.
Similar to graphite, van der Waals interactions hold the layers together; however, the 2D-layers in WSe2 are not atomically thin.
[10] Heating thin films of tungsten under pressure from gaseous selenium and high temperatures (>800 K) using the sputter deposition technique leads to the films crystallizing in hexagonal structures with the correct stoichiometric ratio.
[11] The potential applications of transition metal dichalcogenides in solar cells and photonics are often discussed.
[14] WSe2 photoelectrodes are stable in both acidic and basic conditions, making them potentially useful in electrochemical solar cells.