Tungsten hexacarbonyl
[2] Like its chromium and molybdenum analogs, this colorless compound is noteworthy as a volatile, air-stable derivative of tungsten in its zero oxidation state.
As described in a 2023 survey of methods "most cost-effective routes for the synthesis of group 6 hexacarbonyls are based on the reduction of the metal chlorides (CrCl3, MoCl5 or WCl6) with magnesium, zinc or aluminium powders... under CO pressures".
[3]Another means of preparation involves heating iron pentacarbonyl and WCl6, resulting in the formation of ferrous chloride.
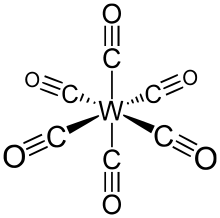
[5] W(CO)6 adopts an octahedral geometry consisting of six rod-like CO ligands radiating from the central W atom with dipole moment 0 debye.
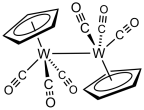
Treatment of tungsten hexacarbonyl with sodium cyclopentadienide followed by oxidation of the resulting NaW(CO)3(C5H5) gives cyclopentadienyltungsten tricarbonyl dimer.