Tyrosine
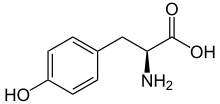
L-Tyrosine or tyrosine (symbol Tyr or Y)[2] or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins.
[7] Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality.
Similar functionality is also presented in serine and threonine, whose side chains have a hydroxy group, but are alcohols.
[9] Phosphorylation of the hydroxyl group can change the activity of the target protein, or may form part of a signaling cascade via SH2 domain binding.
It varies depending on an estimate method, however the ideal proportion of these two amino acids is considered to be 60:40 (phenylalanine:tyrosine) as a human body has such composition.
[12] Tyrosine, which can also be synthesized in the body from phenylalanine, is found in many high-protein food products such as meat, fish, cheese, cottage cheese, milk, yogurt, peanuts, almonds, pumpkin seeds, sesame seeds, soy protein and lima beans.
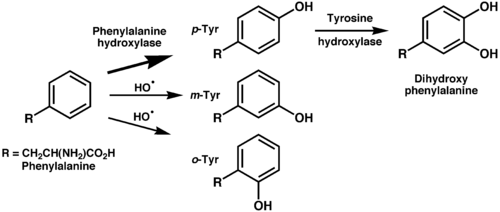
Mammals synthesize tyrosine from the essential amino acid phenylalanine (Phe), which is derived from food.
This enzyme catalyzes the reaction causing the addition of a hydroxyl group to the end of the 6-carbon aromatic ring of phenylalanine, such that it becomes tyrosine.
Tyrosine phosphorylation is considered to be one of the key steps in signal transduction and regulation of enzymatic activity.
Tyrosine (or its precursor phenylalanine) is needed to synthesize the benzoquinone structure which forms part of coenzyme Q10.
The positional description para, abbreviated p, mean that the hydroxyl group and side chain on the phenyl ring are across from each other (see the illustration below).
The m-tyr and o-tyr isomers, which are rare, arise through non-enzymatic free-radical hydroxylation of phenylalanine under conditions of oxidative stress.
[30][31][32] A 2015 systematic review found that "tyrosine loading acutely counteracts decrements in working memory and information processing that are induced by demanding situational conditions such as extreme weather or cognitive load" and therefore "tyrosine may benefit healthy individuals exposed to demanding situational conditions".
The first involves the extraction of the desired amino acid from protein hydrolysates using a chemical approach.