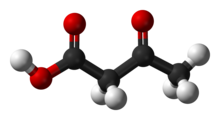
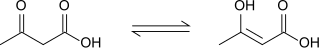
Acetoacetic acid
The methyl and ethyl esters, which are quite stable, are produced on a large scale industrially as precursors to dyes.
Its esters are produced analogously via reactions between diketene and alcohols,[3] and acetoacetic acid can be prepared by the hydrolysis of these species.
Acetoacetic acid displays keto-enol tautomerisation, with the enol form being partially stabilised by extended conjugation and intramolecular H-bonding.
[9] Acetoacetic esters are used for the acetoacetylation reaction, which is widely used in the production of arylide yellows and diarylide dyes.
[3] Although the esters can be used in this reaction, diketene also reacts with alcohols and amines to the corresponding acetoacetic acid derivatives in a process called acetoacetylation.