Urea nitrate
Urea nitrate is a fertilizer-based high explosive that has been used in improvised explosive devices in Afghanistan, Pakistan, Iraq, and various terrorist acts elsewhere in the world such as in the 1993 World Trade Center bombings.
[2] It has a destructive power similar to better-known ammonium nitrate explosives, with a velocity of detonation between 3,400 m/s (11,155 ft/s) and 4,700 m/s (15,420 ft/s).
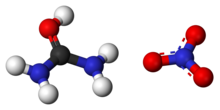
In a simplistic sense, nitric acid dissociates in aqueous solution into protons (hydrogen cations) and nitrate anions.
The electrophilic proton contributed by the acid is attracted to the negatively charged oxygen atom on the urea molecule and the two form a covalent bond.
This is due to the ease of acquiring the materials necessary to synthesize it, and its greater sensitivity to initiation compared to ammonium nitrate based explosives.