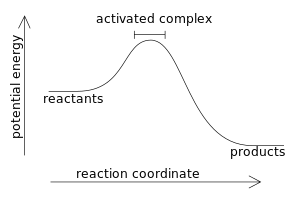
Exothermic reaction
The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system.
Examples are numerous: combustion, the thermite reaction, combining strong acids and bases, polymerizations.
Uncontrolled exothermic reactions, those leading to fires and explosions, are wasteful because it is difficult to capture the released energy.
Nature effects combustion reactions under highly controlled conditions, avoiding fires and explosions, in aerobic respiration so as to capture the released energy, e.g. for the formation of ATP.
The enthalpy change ΔH for a reaction is equal to the heat q transferred out of (or into) a closed system at constant pressure without in- or output of electrical energy.