Vanadium(II) iodide
Vanadium(II) iodide is the inorganic compound with the formula VI2.
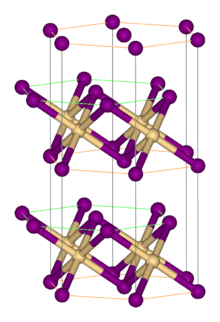
It adopts the cadmium iodide structure, featuring octahedral V(II) centers.
The hexahydrate dehydrates under vacuum to give a red-brown tetrahydrate with the formula V(H2O)4I2.
[2] The original synthesis of VI2 involved reaction of the elements.
[3] It reacts with anhydrous ammonia to give the hexaammine complex.