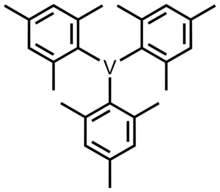
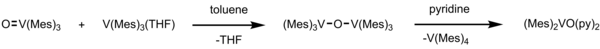Trimesitylvanadium
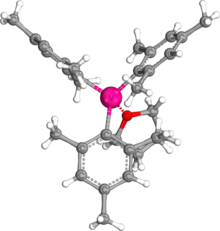
The fact that trimesitylvanadium is recrystallized with THF adduct is due to the strong interaction between vanadium and oxygen.
[4] According to Pyykkö's atomic radii periodic trend, 1.97 Å would be expected for a single bond between vanadium and oxygen.
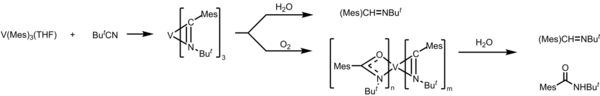
Experiments found that THF in trimesitylvanadium was exchanged with either pyridine or 2,2'-bipyridine when the product was exposed to either chemical.
Since V(Mes)3THF is air- and water-sensitive, when the product from the insertion of tBuCN is exposed to water and/or O2, it undergoes reductive elimination to form imine and amine.
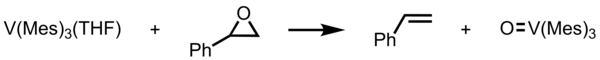
[8][9][10] In vanadium(III) species, V(Mes)3(THF) undergoes deoxygenation of styrene oxide.
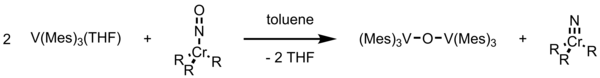
This unique compound has a magnetic moment of 1.65 μB per vanadium at 288 K and a V-O-V stretch vibration of 680 cm−1.
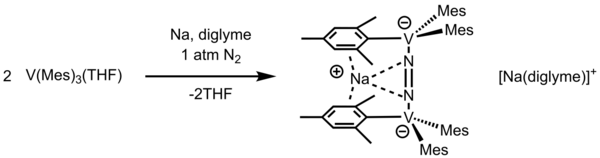
This reaction was also observed with K metal, resulting in the product with a magnetic moment of 1.83 μB per vanadium atom at 293 K. Trimesitylvanadium is a precursor for organometallic fragments in hexagonally packed mesoporous silica (HMS) as a hydrogen storage source.