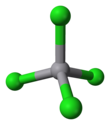
Vanadium tetrachloride
This reddish-brown liquid serves as a useful reagent for the preparation of other vanadium compounds.
[1] Consistent with its high oxidizing power, VCl4 reacts with HBr at -50 °C to produce VBr3.
For example, it converts phenol into a mixture of 4,4'-, 2,4'-, and 2,2'-biphenols:[3] VCl4 is a catalyst for the polymerization of alkenes, especially those useful in the rubber industry.
The underlying technology is related to Ziegler–Natta catalysis, which involves the intermediacy of vanadium alkyls.
VCl4 is a volatile, aggressive oxidant that readily hydrolyzes to release HCl.