Oxidative coupling
[1][2] Many such couplings utilize dioxygen as the stoichiometric oxidant but proceed by electron transfer.
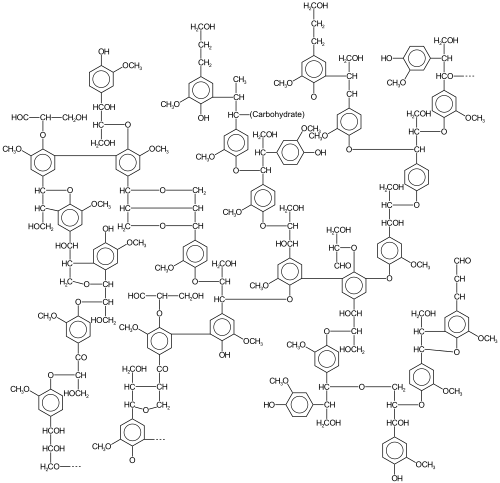
Early examples involve coupling of terminal alkynes:[4] In oxidative aromatic coupling the reactants are electron-rich aromatic compounds.
Typical substrates are phenols and typical catalysts are copper and iron compounds and enzymes,[6] although Scholl demonstrated that high heat and a Lewis acid suffice.
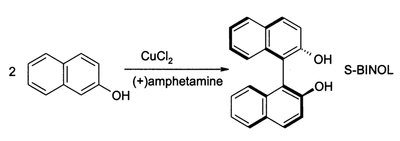
[7] Another reaction is the synthesis of 1,1'-Bi-2-naphthol from 2-naphthol by iron chloride, discovered in 1873 by Alexander Dianin[8] (S)-BINOL can be prepared directly from an asymmetric oxidative coupling of 2-naphthol with copper(II) chloride.
The oxidative coupling of methane gives ethylene:[10][11] The oxygen evolution reaction entails, in effect, the oxidative coupling of water molecules to give O2.