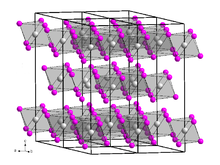
Vanadium(III) bromide
In terms of its structure, the compound is polymeric with octahedral vanadium(III) surrounded by six bromide ligands.
VBr3 has been prepared by treatment of vanadium tetrachloride with hydrogen bromide: The reaction proceeds via the unstable vanadium(IV) bromide (VBr4), which releases Br2 near room temperature.
When heated to temperatures of around 500 °C, a violet gas phase is formed, from which, under suitable conditions, red vanadium(IV) bromide can be separated by rapid cooling, which decomposes at −23 °C.
[2] Like vanadium(III) chloride, vanadium(III) bromide forms red-brown soluble complexes with dimethoxyethane and THF, such as mer-VBr3(THF)3.
Evaporation of these solutions give the salt trans-[VBr2(H2O)4]Br.(H2O)2.