Vapor pressure
It relates to the balance of particles escaping from the liquid (or solid) in equilibrium with those in a coexisting vapor phase.
[2] The most accurate results are obtained near the boiling point of the substance; measurements smaller than 1kPa are subject to major errors.
Better accuracy is achieved when care is taken to ensure that the entire substance and its vapor are both at the prescribed temperature.
In a medical context, vapor pressure is sometimes expressed in other units, specifically millimeters of mercury (mmHg).
The Antoine equation[3][4] is a pragmatic mathematical expression of the relation between the vapor pressure and the temperature of pure liquid or solid substances.
It is obtained by curve-fitting and is adapted to the fact that vapor pressure is usually increasing and concave as a function of temperature.
The Antoine equation has poor accuracy with any single parameter set when used from a compound's melting point to its critical temperature.
Accuracy is also usually poor when vapor pressure is under 10 Torr because of the limitations of the apparatus[citation needed] used to establish the Antoine parameter values.
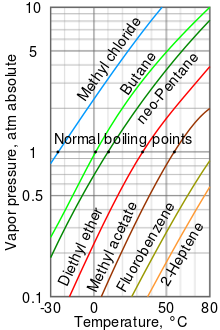
As a general trend, vapor pressures of liquids at ambient temperatures increase with decreasing boiling points.
For example, at any given temperature, methyl chloride has the highest vapor pressure of any of the liquids in the chart.
A nearly straight line is obtained when the logarithm of the vapor pressure is plotted against 1/(T + 230)[8] where T is the temperature in degrees Celsius.
Raoult's law is applicable only to non-electrolytes (uncharged species); it is most appropriate for non-polar molecules with only weak intermolecular attractions (such as London forces).
Because the azeotrope's vapor pressure is higher than predicted by Raoult's law, it boils at a temperature below that of either pure component.
Such a deviation is evidence for stronger intermolecular attraction between the constituents of the mixture than exists in the pure components.
One method is to estimate the sublimation pressure from extrapolated liquid vapor pressures (of the supercooled liquid), if the heat of fusion is known, by using this particular form of the Clausius–Clapeyron relation:[9] where: This method assumes that the heat of fusion is temperature-independent, ignores additional transition temperatures between different solid phases, and it gives a fair estimation for temperatures not too far from the melting point.
Dühring's rule states that a linear relationship exists between the temperatures at which two solutions exert the same vapor pressure.
The following table is a list of a variety of substances ordered by increasing vapor pressure (in absolute units).
Several empirical methods exist to estimate the vapor pressure from molecular structure for organic molecules.
[16] According to the American Meteorological Society Glossary of Meteorology, saturation vapor pressure properly refers to the equilibrium vapor pressure of water above a flat surface of liquid water or solid ice, and is a function only of temperature and whether the condensed phase is liquid or solid.
[18] Equilibrium vapor pressure does not require the condensed phase to be a flat surface; it might consist of tiny droplets possibly containing solutes (impurities), such as a cloud.
[19][18] However, these terms are used inconsistently, and some authors use "saturation vapor pressure" outside the narrow meaning given by the AMS Glossary.
[18] Actually, as stated by Dalton's law (known since 1802), the partial pressure of water vapor or any substance does not depend on air at all, and the relevant temperature is that of the liquid.